Cervical Intraepithelial Neoplasm: Understanding Diagnosis, Treatment, and Prevention
– Cervical dysplasia is a precancerous condition where abnormal cells grow on the surface of the cervix.
– Another name for cervical dysplasia is cervical intraepithelial neoplasia (CIN).
– Most people with cervical dysplasia do not develop cancer.
– Cervical dysplasia is classified on a scale from one to three, with CIN 1 affecting about one-third of the thickness of the epithelium, CIN 2 affecting about one-third to two-thirds of the epithelium, and CIN 3 affecting more than two-thirds.
– Cervical dysplasia primarily affects sexually active individuals assigned female at birth (AFAB) who have a cervix.
– It is most common among women of childbearing age, particularly aged 25 to 35.
– Approximately 250,000 to 1 million cisgender women in the U.S. are diagnosed with cervical dysplasia each year.
– Cervical intraepithelial neoplasia (CIN) is the abnormal growth of cells on the surface of the cervix that could potentially lead to cervical cancer. CIN is graded on a 1-3 scale, with 3 being the most abnormal.
– Human papillomavirus (HPV) infection is necessary for the development of CIN. Many women with HPV infection never develop CIN or cervical cancer. Typically, HPV resolves on its own. However, those with an HPV infection that lasts more than one or two years have a higher risk of developing a higher grade of CIN.
– Most cases of CIN either remain stable or are eliminated by the person’s immune system without the need for intervention. However, a small percentage of cases progress to cervical cancer if left untreated.
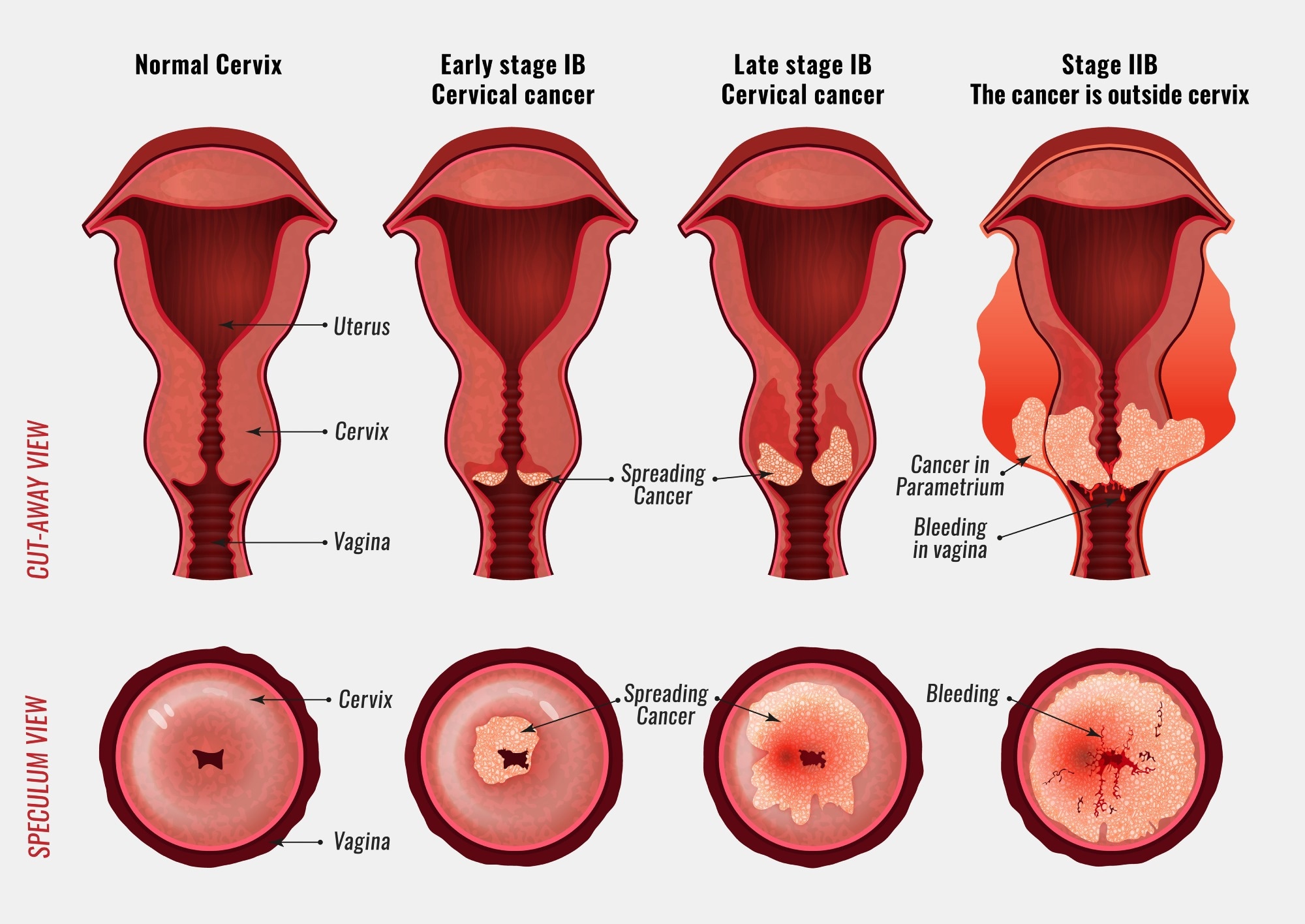
– There are no specific symptoms of CIN alone, but signs and symptoms of cervical cancer may include abnormal bleeding, abnormal discharge, changes in bladder or bowel function, pelvic pain, or abnormal appearance or palpation of the cervix.
– The cause of CIN is chronic infection of the cervix with HPV, especially infection with high-risk HPV types 16 or 18.
– Risk factors for developing CIN include infection with high-risk types of HPV, immunodeficiency, poor diet, multiple sex partners, lack of condom use, and cigarette smoking.
– Cervical intraepithelial neoplasia (CIN) is commonly associated with infection by human papillomavirus (HPV).
– Most women with HPV infection do not develop high-grade intraepithelial lesions or cancer.
– There are over 100 different types of HPV, with approximately 40 known to affect the anogenital area.
– The Digene HPV test is a highly accurate test for HPV, serving as both a direct diagnosis and adjuvant to the Pap smear.
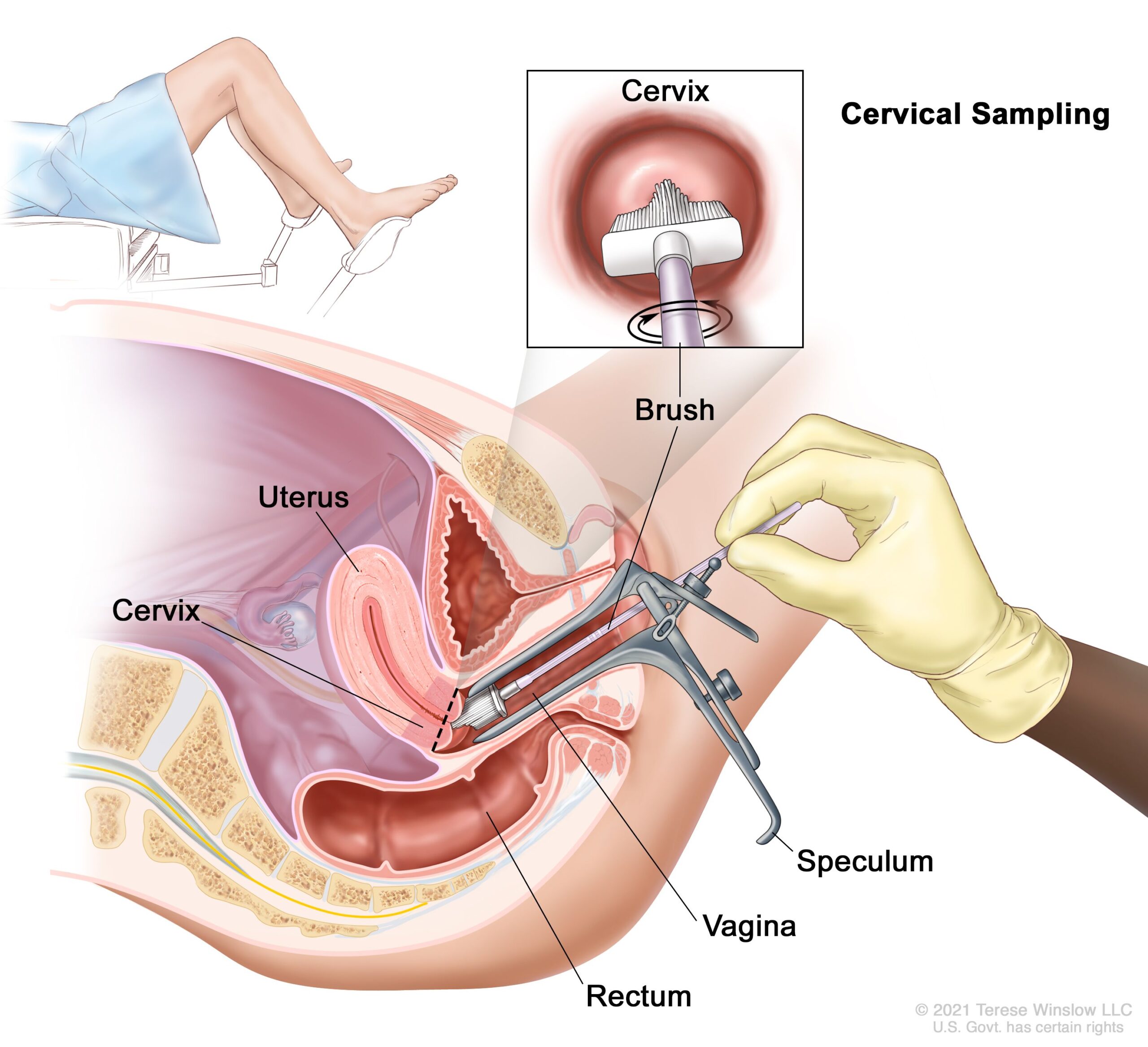
– A colposcopy with directed biopsy is the standard for detecting CIN.
– Diagnosis of CIN or cervical carcinoma requires a biopsy for analysis.
– The Bethesda System for Reporting Cervical/Vaginal Cytologic Diagnoses provides a uniform way to describe abnormal epithelial cells.
– CIN is classified into grades: CIN 1 (mild dysplasia), CIN 2 (moderate dysplasia), and CIN 3 (severe dysplasia)
– CIN 3 can also be referred to as cervical carcinoma in situ.
– Locations of CIN findings can be described in terms of quadrants or clock face positions.
– Cervical intraepithelial neoplasm (CIN) is classified as LSIL or HSIL based on its severity.
– Screening for CIN can be done through Pap smear or testing for HPV.
– The accuracy of Pap smear results can vary.
– Abnormal Pap smear results may lead to colposcopy, which involves examining the cervix under magnification and taking a biopsy.
– HPV testing can identify high-risk HPV types responsible for CIN.
– HPV vaccination is the primary prevention method for CIN and cervical cancer, but it does not protect against all types of HPV known to cause cancer.
– Appropriate management and treatment are used as secondary prevention for cervical cancer cases.
– Treatment for CIN 1 is not recommended if it lasts fewer than two years, as it may clear on its own. Instead, close monitoring is advised.
– Treatment for higher-grade CIN involves removal or destruction of the abnormal cells.
– Retinoids may be effective in causing regression of CIN2.
– Therapeutic vaccines are being tested in clinical trials.
– The lifetime recurrence rate of CIN is about 20%.
– Surgical treatment of CIN may increase the risk of infertility or subfertility.
– Women receiving treatment for CIN during pregnancy may have an increased risk of premature birth.
– People with HIV and CIN 2+ should be managed according to general recommendations.
– Most cases of CIN spontaneously regress. Left untreated, about 70% of CIN 1 will regress within one year and 90% within two years. About 50% of CIN 2 cases will regress within two years. Progression to cervical carcinoma in situ (CIS) occurs in approximately 11% of CIN 1 and 22% of CIN 2 cases. Progression to invasive cancer occurs in approximately 1% of CIN 1, 5% of CIN 2, and at least 12% of CIN 3 cases.
– Treatment does not affect the chances of getting pregnant but is associated with an increased risk of miscarriage in the second trimester.
– Between 250,000 and 1 million American women are diagnosed with CIN annually.
– The estimated annual incidence of CIN in the United States is 4% for CIN 1 and 5% for CIN 2 and CIN 3.