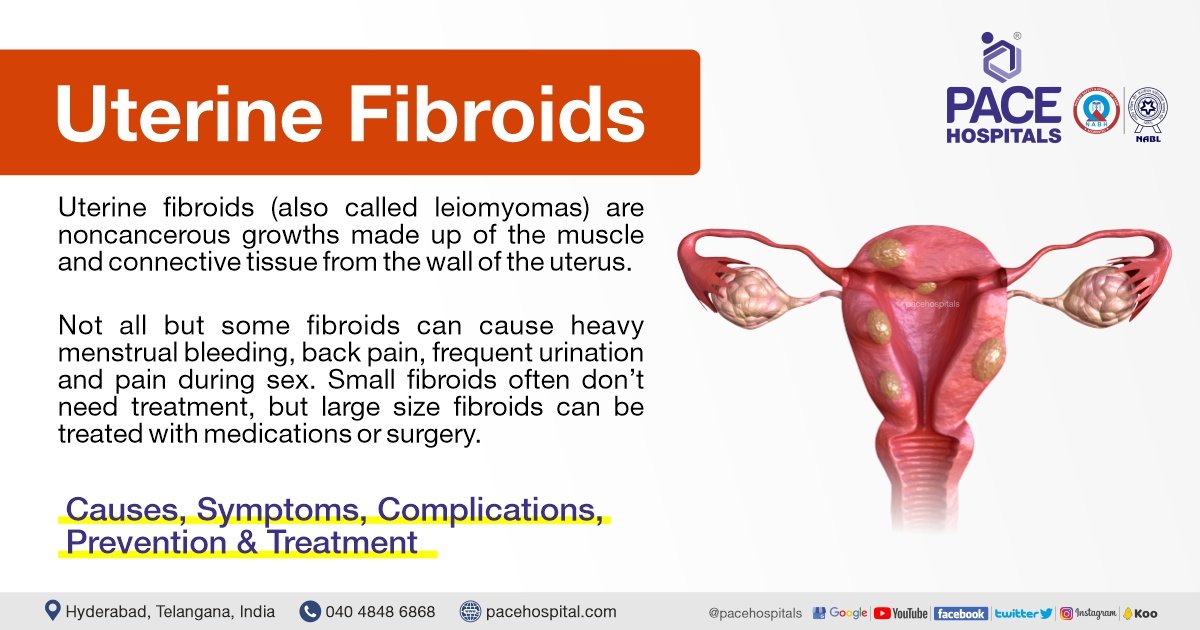
– Uterine fibroids are non-cancerous tumors that grow in the uterus, also known as myomas.
– Myomas are smooth, non-cancerous tumors made partly of muscle tissue that may develop in or around the uterus.
– Myomas in the larger, upper part of the uterus are called fibroids or leiomyomas.
– Most myomas can be seen or felt during a pelvic examination and those causing symptoms can be removed surgically or through less invasive procedures.
– The most common symptom of myomas is vaginal bleeding, which may be irregular or heavy.
– Other symptoms may include heavy bleeding, anemia, fatigue, weakness, painful intercourse, pain, bleeding, or discharge from the vagina if myomas become infected, pressure or lump in the abdomen, difficulties urinating, and urinary tract infections.
– Uterine fibroids, or myomas, affect 20 percent of women in their childbearing years.
– Uterine fibroids can cause abnormal bleeding, pelvic masses, pelvic pain, infertility, and pregnancy complications.
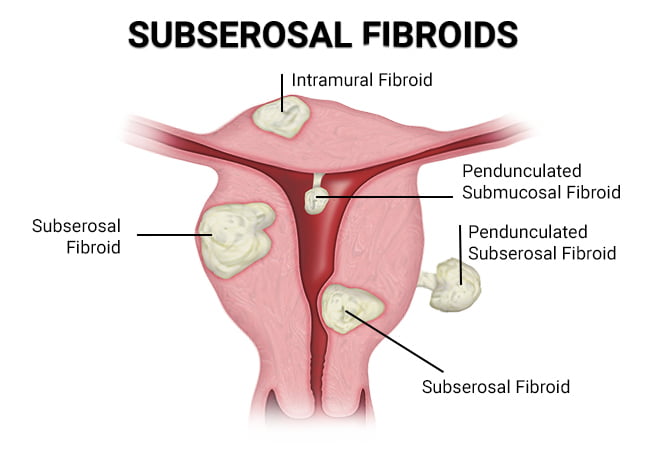
– There are five types of uterine fibroids: intramural, subserosal, submucosal, pedunculated, and intracavitary fibroids.
– Between 70 and 80 percent of women develop a fibroid tumor by the age of 50.
– Estrogen seems to activate the growth of uterine fibroids, and they usually shrink after menopause.
– Hormone therapy after menopause may cause fibroid symptoms to continue.
– African American women are more susceptible to developing fibroids.
– Fibroids tend to grow faster in white women younger than 35 years compared to those older than 45 years.
– Delaying pregnancy until age 30 or older increases the risk of developing fibroids.
– Early menstruation increases the risk of developing fibroids.
– Alcohol and caffeine intake may increase the risk of developing fibroids.
– Specific genetic alterations are linked to fibroid growth.
– Obesity and high blood pressure may play a role in fibroid development and growth.
– A diet rich in red meat may increase the chance of developing fibroids.
– Treatment options for uterine fibroids vary depending on the severity of symptoms and the patient’s desire to have children.
– Hormonal contraception, intrauterine devices, antifibrinolytic drugs, and nonsteroidal agents are options for managing heavy bleeding.
– Endometrial ablation can be performed if the patient does not want to have children.
– Gonadotropin-releasing hormone agonists can shrink fibroids, but they may grow back.
– Myomectomy is a procedure that removes fibroids while preserving the uterus, but fibroids may grow back.
– MRI-guided ultrasound surgery can shrink fibroids and reduce heavy bleeding.
– Uterine fibroid embolization is a minimally invasive procedure that blocks the blood supply to fibroids, causing them to shrink and die.
– Myomas can cause symptoms such as abdominal pain and heavy menstrual bleeding, but some people may remain symptom-free.
– The cause of myomas is unclear, but risk factors include a family history of myoma, obesity, and age.
– More than half of all people with a uterus will experience a myoma by age 50.
– Myomas can vary in size, from as small as a pea to as large as a melon.
– They can be located inside the uterus (intramural myomas), on the outside of the uterine wall (subserosal myomas), have a stalk or stem attaching them to the uterus (pedunculated myomas), or be found just under the lining of the uterus (submucosal myomas).
– Large myomas are considered to be 10 centimeters (cm) or more in diameter.
– Emergency room visits for myoma symptoms, such as pelvic pain and heavy bleeding, have increased from 2006 to 2017, according to a recent study.
– Myomas of the uterus are noncancerous tumors that can cause a variety of symptoms.
– Symptoms may include heavy or prolonged periods, bleeding between periods, pelvic pain, abdominal pressure, a feeling of fullness in the lower abdomen, constipation or diarrhea, frequent urination or trouble urinating, pain during sex, lower back pain, reproductive issues, fatigue, and weakness.
– Myomas are a top cause of hysterectomy surgeries, which come with their own risks and complications.
– The exact cause of myomas is unknown, but they are likely associated with hormone activity.
– High levels of estrogen and progesterone may stimulate their growth, and myomas tend to shrink when hormone levels decrease after menopause.
– Risk factors for developing myomas include a family history of myomas, obesity, high blood pressure, age, and dietary factors such as a diet high in red meat or vitamin D deficiency.
– Myomas are more common among Black individuals with a uterus, and factors such as low vitamin D levels, obesity, stress, genetics, and unequal access to healthcare have been proposed as potential risk factors.
– Medical tests used to diagnose myomas include pelvic examination, ultrasound or transvaginal ultrasound, and magnetic resonance imaging (MRI).
– Treatment for myomas depends on factors such as the severity of symptoms, the size and location of the myomas, the desire for future pregnancy, age, and proximity to menopause.
– Medications can be used to treat myomas, including over-the-counter pain medications, iron supplements for depleted iron levels, and birth control methods to control heavy menstrual bleeding.
– GnRH agonists (hormone-stimulating medications) can be used to temporarily shrink myomas, especially if surgery is planned.
– Surgical options for myomas include laparoscopic myomectomy and uterine fibroid embolization (UFE).
– No specific figures or statistics are mentioned in the article.
– One treatment option is a radiology procedure that uses injections to block blood flow to the myomas, causing them to shrink and sometimes die.
– Another option is MRI-guided ultrasound surgery, which uses ultrasound waves to shrink myomas.
– In more severe cases, other surgical options may be considered.
– Hysterectomy is a surgery to completely remove the uterus, eliminating the fibroids but also making pregnancy impossible in the future.
– Abdominal myomectomy is a surgical procedure that removes the fibroids without removing the uterus, with the possibility of future pregnancy but a risk of the fibroids returning.
– While there are no home remedies that directly treat fibroids, certain complementary therapies like acupuncture, yoga, massage, traditional Chinese medicine, and heating pads may help manage symptoms.
– Lifestyle changes such as dietary changes, exercise, stress management, and weight loss can also be beneficial.
– Myomas can cause complications related to fertility, pregnancy, and childbirth.
– These complications can include fertility issues, pregnancy complications such as miscarriage or early labor, and the need for a cesarean delivery.
– It’s important for those with myomas who want to become pregnant to discuss the condition with their healthcare provider to assess potential risks.
– It is recommended to communicate symptoms affecting one’s life to healthcare providers to determine the most suitable treatments.
Continue Reading